Scientific Calendar April 2019
The fight against malaria starts with diagnostics
How can automated direct detection of malaria-infected red blood cells (RBC) support the global fight against malaria?
Malaria can always be ruled out if a highly sensitive malaria rapid diagnostic test (RDT) is negative
If no malaria-infected RBC are observed on light microscopic review of a peripheral blood smear from a febrile patient, the cause of fever can be assumed to be non-malarial in origin
The automated XN-31 technology enables fast, accurate and precise quantification of malaria-infected RBC, thereby facilitating monitoring of the decrease in parasite load and thus therapeutic efficacy once treatment with anti-malarial drugs has been initiated
Malaria RDTs can be used effectively to monitor the response to anti-malarial drugs
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific background information
Malaria remains one of the most important public health concerns globally, with more than 3.2 billion people in 91 countries at risk of infection [1]. The 2017 WHO world malaria report shows that after a remarkable period of success in global malaria control, the number of cases has once again increased, and progress has stalled. Effort will be needed to reverse this trend, with every case of malaria prevented and death averted contributing to the global drive towards elimination.
Early and accurate diagnosis is a critical aspect of efforts to control malaria. The WHO recommendation is for all suspected malaria cases to have a parasitological test, either microscopy or an RDT, to avoid presumptive therapy based on clinical suspicion alone and to minimise unnecessary exposure to anti-malarial drugs.
Microscopic assessment of a peripheral blood smear enables direct visualisation of parasites in infected red blood cells and is the current diagnostic gold standard. However, it is highly subjective as the limit of detection is greatly influenced by the level of skill and diligence of the microscopist. Consequently, in routine practice, sub-microscopic low-density malaria infection will regularly be missed, with pregnant women being disproportionately affected. Compounding this is the fact that undiagnosed malaria in pregnancy carries a high risk of mortality for both mother and foetus.
On the other hand, immuno-chromatographic rapid diagnostic tests (RDTs) are simple to use and thus are widely deployed as a stand-alone test or as an adjunct to microscopy. RDTs rely on the indirect detection of malaria based on the presence of malarial antigen or protein [2]. There are, however, several drawbacks of RDTs: they cannot distinguish between an acute infection and a recently treated one; they are not quantitative therefore cannot be used to monitor treatment efficacy; not all RDTs detect all Plasmodium species; and they lack specificity (cross-reactivity to other antigens, e.g. rheumatoid factor) and sensitivity (compared to expert microscopy). In anticipation of a greater proportion of low-density infections as malaria control progresses and countries move towards elimination, highly sensitive RDTs, with the P. falciparum histidine-rich protein 2 (HRP2) as the target antigen, have been developed. Unfortunately, the anticipated benefit of enhanced sensitivity has been tempered by the discovery of HRP2 mutations in P. falciparum, the most common and deadliest of the malaria parasites. Consequently, any HRP2-based RDTs will become less efficacious as these mutations become more widespread.
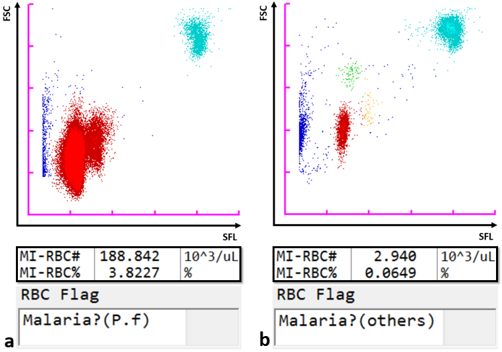
By virtue of its semiconductor laser with a 405 nm beam, which permits the detection of smaller particles, the Sysmex XN-31 automated haematology analyser, in addition to the standard full blood count (CBC) parameters, can detect and count malaria-infected red blood cells (MI-RBC) utilising the principle of fluorescence flow cytometry. RBC are partially permeabilised, permitting the uptake of a fluorescence marker, which stains malaria parasite nucleic acids within infected cells. The side fluorescence light (SFL) intensity and forward scattered light (FSC) signal are plotted to generate a malaria (M) scattergram. The size of the infected RBC and stainable nucleic acid content increase as ring forms develop sequentially from trophozoites and schizonts during the asexual life cycle. Likewise, gametocytes, which are formed after a developmental trigger to sexual reproduction, are uniquely identifiable. Thus, the XN-31 categorises malaria infection within a sample by life cycle stages based on the SFL, reflecting the amount of nucleic acid, and the FSC, reflecting the size of MI-RBC. The scattergram patterns differ amongst Plasmodia species, which is used by the analyser software to give an indication of the likely species (P. falciparum or other).
The analyser has a lower limit of detection of 20 parasites/μL, which is well below that of regular RDTs and routine smear microscopy (≥100 parasites/μL [3]).
The XN-31 analyser is an ideal modality for the precise recognition and rapid automated enumeration of malaria parasitaemia, independent of the skill of the operator or species involved. It detects the actual parasite and not any by-product, such as antigens, haemozoin or phagocytosed parasites within white blood cells, making it more suitable for malaria detection compared to RDTs and other indirect automated methods [4]. Furthermore, concurrent measurement of a CBC is a unique feature that provides valuable data for clinical correlation.
Scattergrams
Analysis results including scattergrams, MI-RBC values and the species RBC flags for patient samples infected with a) P. falciparum (P.f) and b) P. ovale (others). P. ovale parasites were confirmed microscopically.
Legend: SFL: side fluorescence light; FSC: forward scattered light; blue dots: non-infected RBC, platelets and debris; red dots: MI-RBC; green and orange dots: different P. ovale life cycle forms; turquoise dots: white blood cells
References
- WHO. World malaria report 2018. Geneva: World Health Organization; 2018. www.who.int/malaria/publications/world-malaria-report-2018/en/.
- Chotivanich K, Silamut K, Day N. Laboratory diagnosis of malaria infection ‒ A short review of methods. N Z J Med Lab Sci. 2007; 61:4-7.
- WHO. Policy brief on malaria diagnostics in low transmission settings. Geneva: World Health Organisation; 2014. www.who.int/malaria/publications/atoz/malaria-diagnosis-low-transmission-settings-sep2014.pdf
- Pillay E, Khodaiji S, Bezuidenhout BC, Litshie M, Coetzer TL. Evaluation of automated malaria diagnosis using the Sysmex XN-30 analyser in a clinical setting. Malar J. 2019; Jan 22; 18(1):15. doi: 10.1186/s12936-019-2655-8.