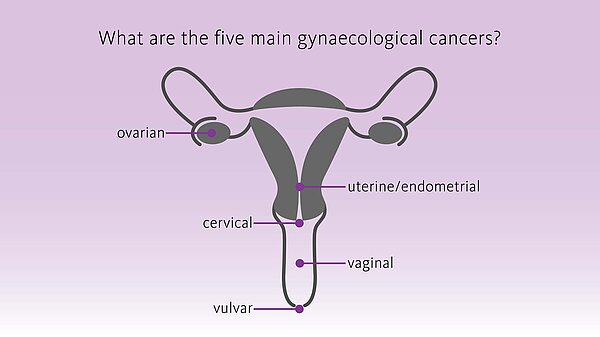
Diagnostic solutions in gynaecological cancers - precisely bring to light what needs to be seen
Malignancies affecting the female reproductive organs are collectively called ‘gynaecological cancers’. They account for about 1,4 million new cases each year and are responsible for about 700,000 deaths annually worldwide, with cervical, endometrial and ovarian cancers being the most frequently diagnosed. [1]
Detection of gynaecological cancers at an early stage, their accurate diagnosis and staging as well as treatment play a key role for prognosis/outcome and patient’s quality of life.
During the last years, the diagnostic and surgical approaches have become more personalised and less invasive. This is also due to the establishment of the sentinel lymph node concept and to the increased application of molecular genetic testing for diagnosis which allows a better characterisation and understanding of the cancer, its behaviour and aggressiveness as well as patient’s risk and prognosis, thus supporting the choice of the right treatment.
We offer a range of solutions based on cutting-edge molecular biology technologies supporting healthcare professionals and patients along the whole pathway of care.
A more flexible staging standard
During treatment of many gynaecological cancers, it is important to assess whether the cancer has spread to the lymph nodes. The first lymph nodes where cancer cells are most likely to spread to are called ‘sentinel lymph nodes’ (SLNs). A sentinel lymph node biopsy will help determine the ‘stage’ of the cancer and thus the individual treatment plan, if needed. Using the Sentimag® - Magtrace® magnetic localisation system, SLN can be identified effectively while avoiding the issues associated with radioactive materials. [2-3]
The procedure
To be able to identify the SLNs during surgery, the surgeon will inject a small amount (just up to 2 ml) of Magtrace® tracer close to the cancer. After injection, the Magtrace® tracer will flow through the lymphatic system and will ‘mark’ the SLNs.
The injection may be administered to 30 days prior to surgery or in the operating theatre after following anaesthesia. During the surgical procedure, the surgeon will use the Sentimag® probe to identify the marked SLNs, which will be removed and sent to the pathology lab for analysis.
Benefit
The Magtrace® tracer is an established technique that has been used in over 65,000 surgical procedures worldwide. It enables greater flexibility and convenience for both patients and physicians, as it can be injected within a 30-day window.
Endomag®, Sentimag® and Magseed® are registered European Union trademarks of Endomagnetics Ltd
Magtrace® is a registered trademark of Endomagnetics Ltd in the United Kingdom
Cancer diagnosis with OSNA
Cancer staging is the step that determines the type and extent of treatment of a patient. An essential part of this staging process is an assessment of the nodal status.
Revealing the whole picture
Lymph node status is the most important prognostic factor and staging parameter in gynaecological cancer management. Whether lymph nodes are involved or not, it guides clinicians in their decision about the suitable surgical approach, the extent of the surgical intervention and what kind of additional therapy might be necessary.
Current histopathological methods have several limitations that either do not allow the provision of reliable diagnostic information in an intraoperative scenario or lack sensitivity for accurate staging. As a result, there is a risk that small metastases remain undetected leading to false negative results and inadequate treatment.
In contrast, OSNA (one step nucleic acid amplification) allows fast, sensitive and standardised molecular lymph node assessment, enabling accurate determination of the nodal status. By analysing the entire lymph node, the OSNA method provides accurate staging and a reliable basis for patient tailored treatment decisions, even during an operation, if necessary – thus helping to prevent under- or overtreatment and to improve the patient’s quality of life.
Treatment solutions in gynaecological cancers
Ovarian cancer is usually detected at later stage and is a leading cause of death from gynecological cancers. The treatment for this type of cancer has improved over the last 30- years due to better surgical approaches and improved availability of chemotherapeutic drugs. This is especially so since the introduction of platinum-based drugs and, more recently, the addition of taxanes. Despite these improvements in treatment, up to 30% of patients show no clinical remission and the majority of women will eventually relapse generally with an incurable disease. [4] Although effective surgery and treatment of the disease at an early stage are associated with an improved survival, it is impossible to predict which patient will progress or relapse during or after chemotherapy. This prediction is essential since patients who are resistant might benefit from a different combination of treatment [4] including modern targeted therapy.
The development of genetic testing has allowed a better characterisation and understanding of ovarian cancer, its behaviour and aggressiveness as well as the patient’s risk and prognosis, thus supporting the choice of the right treatment.
Germline mutations of the BRCA1 and BRCA2 genes lead to a high lifetime risk of ovarian cancer. They represent the predominant and most well-characterised genetic risk factors so far identified for the disease. BRCA1 and BRCA2 are involved in almost half of all families with two or more cases of ovarian cancer. As the BRCA1 and BRCA2 genes encode the proteins that are involved in DNA repair, tumours with alterations in either gene are particularly sensitive to specific anticancer agents that act by damaging DNA. [5]
Reliable assessment of the tumour’s mutational status is decisive in selecting cancer patients eligible for targeted therapy. To investigate such genetic changes, oncologists can make use of a variety of molecular genetic tests which may support them in making personalised treatment decisions. Precision diagnostics are key to selecting the right therapy at the right time for the right cancer patient.
Molecular tests for therapy selection
CytoCell FISH (Fluorescent In Situ Hybridisation) probes targeting genes MET, ROS1, CHD5, TOP2A and ZNF217 are used to aid pathologists and clinicians to understand tumour behaviour/aggressiveness and therefore patient prognosis.
BRCA1, BRCA2, TP53, PTEN, ATM, ATR and NF1 are among the most common germline and somatic mutated genes underlying the pathogenesis of ovarian cancer that can be investigated by next generation sequencing (NGS) tests. The Sysmex solution to help characterise the overall genomic landscape of ovarian cancer is SureSeqTM Ovarian Cancer Panel.
Read two inspiring survivor stories
They share words of inspiration, hope and how their lives have been changed by this disease.
Find out more about how Sysmex makes a difference in the battle to conquer gynaecological cancers
Explore more

References
-
Sung H, et al. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. PMID: 33538338
-
Rzepka et al. (2014): Sentinel lymph node identification using a magnetic tracer for endometrial cancer: A pilot study. J Clin Oncol 32, 2014 (suppl; abstr e16550)
-
Jedryka MA, Klimczak P, Kryszpin M, et al. (2020): Superparamagnetic iron oxide: a novel tracer for sentinel lymph node detection in vulvar cancer. Int J Gynecol Cancer, doi:10.1136/ ijgc-2020-001458
-
Helleman J. et al., (2006) Molecular profiling of platinum resistant ovarian cancer. Int J Cancer 118(8):1963-71.
-
Radu MR et al. (2021) Ovarian Cancer: Biomarkers and Targeted Therapy. Biomedicines. Jun 18;9(6):693. doi: 10.3390/biomedicines9060693.