Scientific Calendar March 2021
Early recognition of diabetic nephropathy
Which factors are key promotors of diabetic nephropathy?
Persistent hyperglycaemia
Lipaemia
Inflammation
Systemic hypertension
Auto-immune response
Glomerular hypertension
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific background
Chronic kidney disease (CKD) is defined as ‘persistent abnormalities of the kidney structure and/or function, present for more than three months’ [1] with hypertension, diabetes, and obesity being the main risk factors for the onset and progression of CKD. Since these civilisation diseases show a steadily increasing prevalence since the 1990s, an increase of the prevalence of CKD to 9.1% is not surprising. Besides the individual effects on CKD patients’ quality of life, CKD is putting a significant burden on healthcare expenditures and the society [2].
Apart from the estimated glomerular filtration rate (eGFR), urinary albumin is a key indicator for the development of CKD. It is essential to distinguish between mildly and severely increased albuminuria. While the early detection of albuminuria at a mildly increased level allows reverting the onset of CKD, severely increased albuminuria is irreversible and a course towards end-stage renal failure is set. [3]
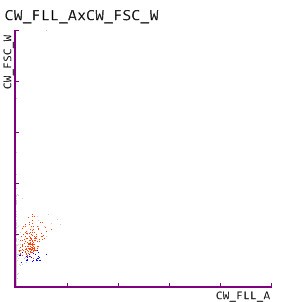
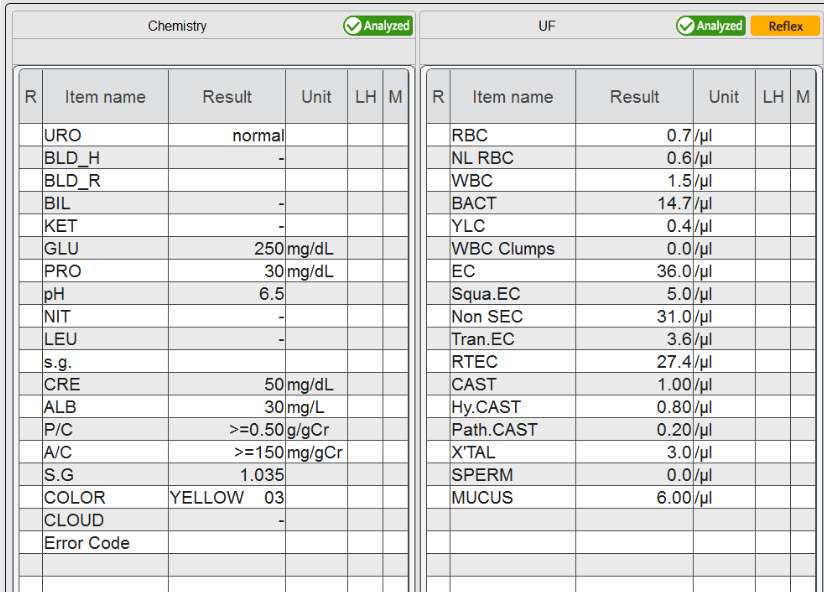
In our current patient case, a 68-year-old patient, who is suffering from a long history of obesity and diabetes mellitus, presented himself to his physician for a routine check-up. Due to persistent hyperglycaemia, test strip analysis of a urine sample was performed, revealing increased levels of urinary albumin and protein, as well as increased albumin:creatinine and protein:creatinine ratios. Persistent albuminuria and proteinuria are clear indicators for an impaired glomerular filtration barrier; therefore an additional analysis of urinary particles was performed, which revealed the presence of renal tubular epithelial cells. The physician suspected a mildly increased albuminuria and slight glomerular damage, indicating the onset of CKD.
The suspected mildly increased albuminuria was finally confirmed by an immunonephelometric assay, and respective measures – including the adjustment of diabetes through close glycaemic control and weight reduction – were taken with the aim to stop and revert the onset of CKD.
In many countries, there is no general monitoring of the onset of CKD caused by civilisation diseases in place. This patient case underlines the importance of a broad CKD screening of risk group patients and offers test strip analysis as a suitable tool for albuminuria monitoring. With the Meditape 11A strips in combination with a UC-Series instrument, for the first time, analytical quality comparable to quantitative immuno-based assays can be obtained from a simple test strip analysis [4].
Test strip-mediated screening for albuminuria has been demonstrated to allow a significant reduction of expensive immuno-based wet chemistry assays and with that a cost reduction [5], further substantiating the potential of test strip-mediated CKD screening of risk groups.
References
[1] KDIGO (2012) Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013; 3:1–150.
[2] GBD Chronic Kidney Disease Collaboration (2020) Global, regional, and national burden of chronic kidney disease 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 395:709–33.
[3] Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013; 3:19–62.
[4] Delanghe JR, Himpe J, De Cock N, Delanghe S, De Herde K, Stove V and Speeckaert MM (2017) Sensitive albuminuria analysis using dye-binding based test strip. Clin Chim Acta 471:107–12.
[5] Salinas M, Lopez-Garrigos M, Flores E, Lugo J and Leiva-Salinas C (2020) Urinary albumin strip assay as a screening test to replace quantitative technology in certain conditions. Clin Chem Lab Med 57(2):204–9.