Scientific Calendar April 2021
CAD treatment with DAPT
What is a common limitation of clopidogrel therapy, thereby influencing the response to the drug?
Polymorphisms of clopidogrel
Polymorphisms of acetylsalicylic acid
Polymorphisms of CYP2C19
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific background
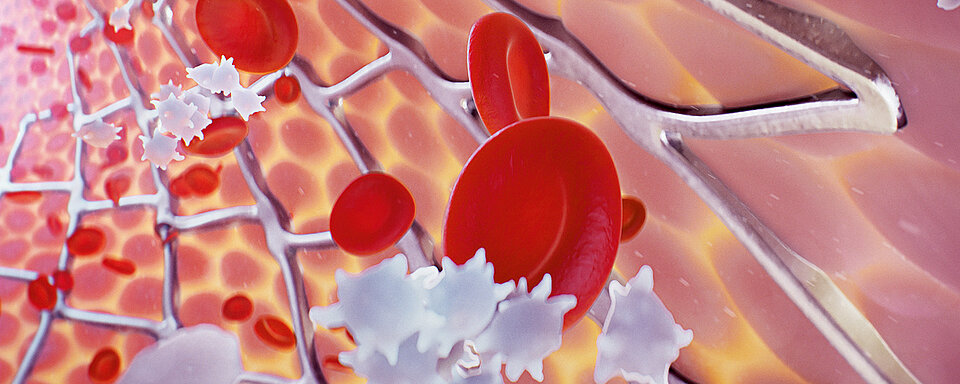
Platelets play an important and primary role in blood coagulation. When a blood vessel is injured, platelets adhere to the side of the vessel wall damage (‘platelet adhesion’) and stick together (‘platelet aggregation’) to prevent further blood loss. Furthermore, platelets release procoagulant substances to promote clot formation (‘secondary haemostasis’). Platelets also play an important role in the development of thrombotic complications in arteries and veins.
Undesirable platelet activation can be caused by vascular injury because of percutaneous coronary intervention (PCI) or occur spontaneously during plaque rupture, triggering a cascade of signalling events and ultimately leading to the development of serious complications, including myocardial infarction. Dual antiplatelet therapy (DAPT) with clopidogrel and acetylsalicylic acid (ASA) aims to prevent arteriothrombotic events in patients who have acute artery disease (CAD) and are undergoing PCI. [1-7]
Superiority of DAPT to ASA therapy alone in reducing adverse events in both short-term and long-term treatments of CAD has been confirmed during several clinical trials. [5–9] However, some patients show an insufficient reduction in platelet reactivity despite treatment with the currently recommended drug doses and are therefore still exposed to an increased risk of thrombosis. [10]
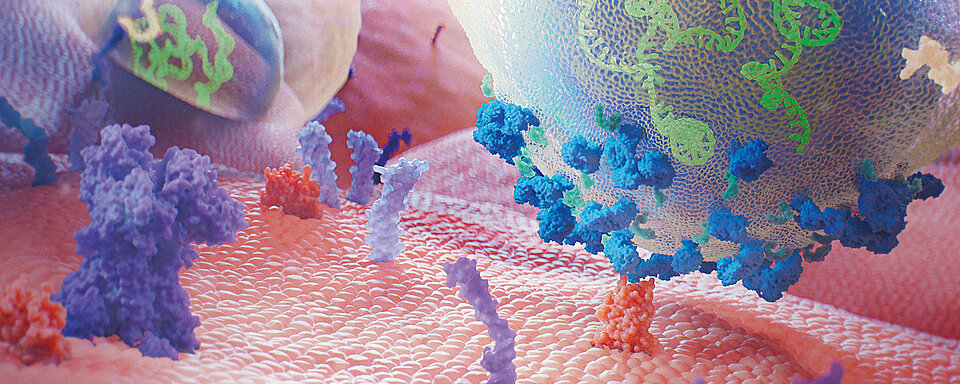
Clopidogrel is a pharmacological substance that must be converted into an active substance (metabolite) before becoming effective. The enzymes responsible for the conversion are the cytochrome P450 (CYP450). The active metabolite is formed primarily by CYP2C19 with the involvement of several other CYP enzymes, including CYP1A2, CYP2B6 and CYP3A4. The active metabolite irreversibly and selectively inhibits the binding of adenosine diphosphate (ADP) to its receptor P2Y12. The subsequent ADP-mediated activation of the glycoprotein (GP) IIb / IIIa complex is thus also inhibited, which then leads to an inhibition of platelet aggregation. In addition, platelet aggregation triggered by other agonists is also inhibited by antagonising the amplification of ADP mediated platelet activation. Since the formation of the active metabolite is dependent on CYP450 enzymes, a genetic polymorphism or the inhibition of the enzyme by other drugs may have a direct influence on the effectiveness of the drug. Polymorphisms, mainly of CYP2C19, cause reduced function and thus an absent or reduced metabolism (slow metaboliser). The recommended starting dose and daily maintenance dose (in combination with aspirin) might be insufficient in slow metaboliser and higher doses are needed to maintain sufficient platelet inhibition and archive a lower incidence of non-responsiveness. [10, 11]
ASA is a member of the acidic, non-steroidal anti-inflammatory drugs with analgesic, anti-pyretic and anti-inflammatory properties. Their mode of action is based on the irreversible inhibition of cyclooxygenase enzymes that are involved in prostaglandin and subsequent thromboxane A2 (TXA2) synthesis in platelets. TXA2 is a physiological, aggregation-promoting substance but plays a role in pathological arteriothrombotic complications as well. Repeatedly administered ASA doses between 20–325 mg lead to a 30–95% inhibition of the enzyme activity. Inhibitory effect may persist in decreasing manner for up to 10 days after drug withdrawal. [12]
Despite the well-documented antithrombotic effects of aspirin, a significant proportion of patients receiving aspirin will experience a (relapse of) cardiovascular event. This observation has led to the term ‘aspirin resistance’. [13]
A distinction must be made between clinical (occurrence of thrombotic events despite aspirin therapy), biochemical (normal laboratory results), pharmacological (still existing TXA2 synthesis) and functional (persistence of platelet aggregation under treatment) aspirin resistance. [13]
The causes of aspirin resistance vary and are not always clear [14]:
- ‘Pseudo-resistance’ due to lack of intake, non-compliance or underdosing
- Resorption disorders, accelerated platelet turnover or alternative routes of thromboxane synthesis
- Interference with other pharmaceuticals, e.g. ibuprofen
- Alternative platelet activation through ADP and collagen
- Decrease in platelet sensitivity to aspirin with long-term therapy
- Genetic polymorphisms
- Underlying disease
- Lifestyle
In some patients, the usual therapy does not sufficiently inhibit platelet aggregation. This may have serious consequences for patients, therefore a reliable method for checking the inhibition of platelet aggregation is of utmost importance.
References
[1] Strukova S. Blood coagulation-dependent inflammation. Coagulation-dependent inflammation and inflammation- dependent thrombosis. Front Biosci 2006; 11: 59–80.
[2] Gurbel PA et al. Platelet activation in myocardial ischemic syndromes. Expert Rev Cardiovasc Ther 2004; 2: 535–545.
[3] Jackson SP, Nesbitt WS, Kulkarni S. Signalling events underlying thrombus formation. J Thromb Haemost 2003; 1: 1602–1612.
[4] Patrono C et al. Platelet-active drugs: The relationships among dose, effectiveness, and side effects. Chest 2001; 119 (1 Suppl): 39S–63S.
[5] Yusuf S et al. Clopidogrel in unstable angina to prevent recurrent events trial investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. New Engl J Med 2001; 345: 494–502.
[6] Mehta SR et al. Clopidogrel in unstable angina to prevent recurrent events (CURE) trial investigators. Effects of pre-treatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001; 358: 527–533.
[7] Steinhubl SR et al. CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: A randomized controlled trial. JAMA 2002; 288:2411–2420.
[8] Marco Valgimigli, Héctor Bueno, Robert A Byrne, Jean-Philippe Collet, Francesco Costa, Anders Jeppsson, Peter Jüni, Adnan Kastrati, Philippe Kolh, Laura Mauri, Gilles Montalescot, Franz-Josef Neumann, Mate Petricevic, Marco Roffi, Philippe Gabriel Steg, Stephan Windecker, Jose Luis Zamorano, Glenn N Levine, ESC Scientific Document Group, ESC Committee for Practice Guidelines (CPG), ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS), European Heart Journal, Volume 39, Issue 3, 14 January 2018, Pages 213–260.
[9] Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020 Aug 29: ehaa575. doi: 10.1093/eurheartj/ehaa575. Epub ahead of print.
[10] Gurbel PA, Tantry US. Aspirin and Clopidogrel Resistance: Consideration and Management. J Interven Cardiol 2006; 19: 439–448.
[11] Clopidogrel – Product Information. Annex 1 – SUMMARY OF PRODUCT CHARACTERISTICS. European Medicines Agency. EMA/EMEA/H/C/000975 - IB/0074. First published: 26.10.2009. Last updated: 02.02.2021.
[12] Aspirin® Direkt – Product Information. SUMMARY OF PRODUCT CHARACTERISTICS. European Medicines Agency.
[13] Gremmel T, Steiner-Böker S, Kopp CW. Aspirinresistenz – Klinische Relevanz eines komplexen Phänomens. Zeitschrift für Gefäßmedizin 2008; 5 (1), 6-10.
[14] Adriana Méndez RH, Huber AR. Aspirinresistenz - ein komplexes, aber ernstzunehmendes Phänomen. Schweiz Med Forum 2006; 6: 898–904.